
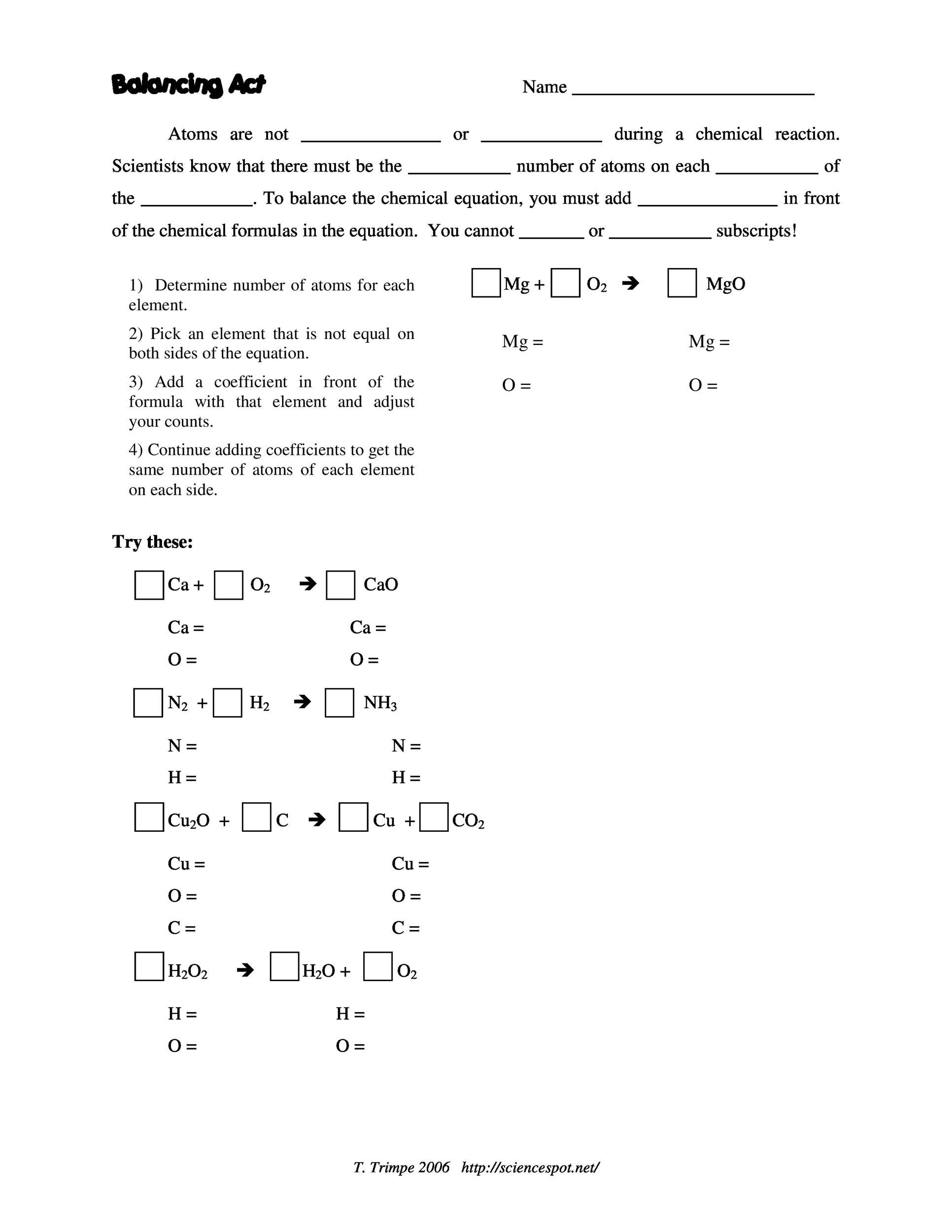
If the elements in a chemical formula are properly capitalized, the smart case converter leaves them as you have typed. The equation can be written in lowercase letters.To enter the equation sign, you can use either "=" or "->" or "→" symbols.To enter charge species, just type them as they are (Al3+, NH4+, SO42-) or explicit declare (Hg2^2+).You can ease off your pressure by using the tool. The tool proved to be very helpful for students. Many students are unable to solve such chemical equations on their own. You will get all the necessary help to solve them quickly. All types of parentheses are correct, for example Na2Zn32*9H2O The Balance Equation Calculator tool comes in handy for students facing problems with chemical equations.Spaces are irrelevant, for example ag no3 is equal agno3 Week 4 for Introduction to Chemistry: Reactions and Ratios is about to begin This week will be filled.A valid equation must have the same elements on both sides of the equation.Write an unbalanced chemical equation in the input field using following rules and click 'Balance' (for example: ca3(po4)2(s) + h2so4(aq) = h3po4(aq) + caso4(s) ). the same number of atoms of each element must exist on the reactant side and the product side of the equation. This is important because a chemical equation must obey the law of conservation of mass and the law of constant proportions, i.e. Write down the chemical equation for the total combustion of methyl alcohol CH 3 OH. Balancing chemical equations involves the addition of stoichiometric coefficients to the reactants and products. Chem 1110 - Chapter 2: Atoms, Molecules, and Ions Practice Quiz 4. The law of conservation of mass states that in an ordinary chemical reaction, matter is neither created nor destroyed, that is, a chemical equation must have the same number of atoms of each element on both sides of the equation. Northrups Chem 111 Section TTU General Chemistry. If, for example, you write 2 H 2 O, that means you have 2 times the number of atoms in each water molecule, which would be 4 hydrogen atoms and 2 oxygen atoms. Coefficients are whole number multipliers. The balanced equation is: 2 Fe 2 O 3 + 3 C 4 Fe + 3 CO 2. Subscripts (small numbers on the right of certain atoms, such as iron and oxygen in this case) are never altered. When balancing equations, you never change subscripts. This is accomplished by altering the compound coefficients (numbers placed in front of compound formulas). There are other methods to balance chemical equations, that’s why these are tips and not rules.

What does it mean to be balanced? It means that the law of conservation of mass is obeyed. Add Coefficients To Balance Mass in a Chemical Equation. Balancing Chemical Equations - Tips & Tricks Remember, when trying to balance equations, you can only change the value of the coefficient in front of the compound or element, not the subscripts. Every chemical equations must be balanced.


 0 kommentar(er)
0 kommentar(er)
